Analysis of The Reason Why Lithium Batteries Explode

You face a real risk when Lithium Batteries Explode. The main reason comes from thermal runaway—a rapid chain reaction that starts if the battery overheats or has an internal problem. This process causes the battery to heat up even more, making it unstable and dangerous. Knowing why these explosions happen helps you handle batteries safely.
Key Takeaways
- Lithium batteries can explode due to thermal runaway, which happens when they overheat. Avoid overcharging to prevent this dangerous reaction.
- Internal short circuits can cause battery explosions. Always handle batteries carefully and avoid physical damage to reduce this risk.
- The electrolyte in lithium batteries is flammable. Use certified chargers and avoid exposing batteries to high temperatures to prevent fires.
- Watch for warning signs like swelling, heat, or unusual noises. If you notice these, stop using the battery immediately for safety.
- Choose high-quality batteries with safety certifications. This helps ensure better performance and reduces the risk of battery failure.
Why Lithium Batteries Explode

Thermal Runaway
You face the greatest risk of battery explosions when thermal runaway occurs. This process starts when the battery cell overheats, often due to overcharging or internal failure. Once the temperature rises, a chain reaction begins. The battery heats up rapidly, and each stage of the reaction makes the situation worse. You can see how this process unfolds in the table below:
| Stage | Initiating temperature | Description |
|---|---|---|
| 1. SEI degradation | ~80°C | The solid electrolyte interphase (SEI) breaks down, exposing the electrode to the electrolyte. |
| 2. Electrolyte decomposition | ~100°C | The electrolyte decomposes, releasing flammable gases and raising the temperature. |
| 3. Separator melting | ~130°C | The separator melts, allowing the electrodes to touch and causing a short circuit. |
| 4. Cathode decomposition | ~150°C | The cathode decomposes, releasing more flammable gases and oxygen, which can cause an explosion. |
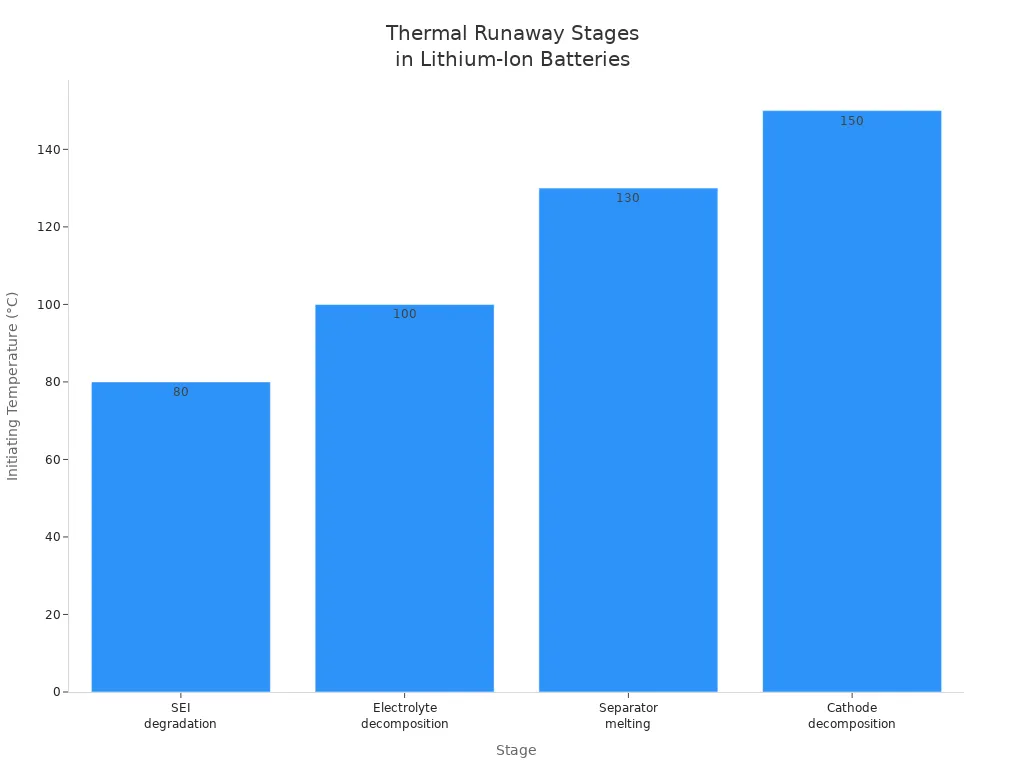
You should know that lithium batteries explode more often than other battery types because they have lower thermal stability. Sodium-ion batteries, for example, react less violently under thermal stress. When thermal runaway starts, the battery releases heat and flammable gases. If the pressure builds up, the cell can burst, leading to battery explosions. Overcharging is a major trigger for this process, as it pushes the battery beyond safe limits.
Internal Short Circuits
Internal short circuits play a key role in why lithium batteries explode. When the separator inside the battery fails, the anode and cathode can touch. This contact creates a direct path for current, causing rapid heating and triggering thermal runaway. You might encounter internal short circuits due to several reasons:
- Mechanical abuse, such as puncturing or compressing the battery, can damage the separator.
- Electrical misuse, including overcharging and dendrite growth, can create bridges between electrodes.
- High temperatures can cause the separator to collapse, leading to a short circuit.
The table below summarizes the main causes:
| Cause of Internal Short Circuit | Description |
|---|---|
| Manufacturing Defects | Copper particles on the cathode can dissolve and deposit on the anode, causing shorts. |
| Mechanical Abuse | Punctures or compression can rupture the separator. |
| Electrical Misuse | Lithium dendrites can pierce the separator. |
| Thermal Misuse | Excessive heat can melt the separator. |
You should always avoid overcharging and physical damage. These actions increase the risk of internal short circuits, which often serve as the initial event in battery explosions. Once a short circuit forms, the battery overheats, releasing flammable gases and sometimes causing the cell to explode.
⚠️ Note: The separator's failure is a leading cause of battery explosions. You should never ignore signs of swelling, heat, or damage in your device.
Flammable Electrolyte
The electrolyte in lithium-ion batteries is a liquid solution that helps ions move between the anode and cathode. This liquid is highly flammable. When the battery overheats, especially during overcharging or a short circuit, the electrolyte can vaporize and catch fire. Most lithium batteries explode because the flammable electrolyte ignites under high temperatures or sparks from internal failures.
You should know that solid-state batteries use a non-flammable solid electrolyte, which greatly reduces the risk of battery explosions. However, most consumer devices still rely on liquid electrolytes, making safety precautions essential. The combination of a flammable electrolyte, high energy density, and the risk of overcharging explains why lithium batteries explode more frequently than other types.
? Tip: Always use certified chargers and avoid overcharging your devices. This simple step can prevent dangerous overheating and reduce the risk of battery explosions.
Common Causes of Battery Explosions
Overcharging
You encounter one of the most common reasons for battery failure when you overcharge a lithium-ion battery. Overcharging causes a rapid increase in voltage, which leads to irreversible changes in the positive electrode's structure. This process generates excessive heat and gas, resulting in a sharp rise in internal pressure. If the pressure exceeds the battery's design limits, the shell can rupture, triggering a short-circuit and thermal runaway. You should understand that overcharging also causes the internal separator to melt or contract, increasing the risk of short-circuit and battery failure.
Overcharging leads to:
- Rapid voltage increase
- Excessive heat and gas generation
- Sharp rise in internal pressure
- Separator melting or contraction
- Short-circuit and thermal runaway
Battery manufacturers implement several safety mechanisms to prevent overcharging. These include protection voltage settings in Battery Management Systems (BMS), material modifications, anti-overcharge additives, voltage-sensitive films, and devices like Over-Pressure Devices (OPD) and Current Interrupt Devices (CID). You should always use certified chargers and avoid leaving devices plugged in overnight to minimize the risk of battery failure.
High Temperatures
Heat is a major factor in battery failure. When you expose lithium-ion batteries to high temperatures, you accelerate internal reactions and increase the risk of thermal runaway. Research shows that batteries begin to lose control over their internal temperature between 60°C and 100°C. This range marks the onset of thermal runaway, which can lead to fires or explosions. You should avoid using or storing batteries in hot environments, such as inside cars or under direct sunlight.
| Temperature (°C) | Description |
|---|---|
| Up to 60 | Cells tolerate heat but may degrade over time |
| Over 45 | Gradual capacity erosion and increased failure risk |
| Above 60 | Speeds up battery aging and poses safety risks |
| 35 to 100 | Affects performance and lifespan |
| Over 100 | Serious risk of battery melting or explosion |
Leaving batteries in hot environments can elevate internal pressure and trigger battery failure. You should always keep devices away from heat sources to prevent dangerous reactions.
Physical Damage
Physical damage is another common reason for battery failure. When you puncture, crush, or drop a lithium-ion battery, you risk damaging the separator and causing a short-circuit. Continued use of damaged batteries increases the likelihood of battery failure and explosion. Physical damage can result in severe injuries, including burns, blast injuries, eye injuries, and traumatic brain injuries.
| Injury Type | Description |
|---|---|
| Burn Injuries | Severe burns, often requiring medical treatment. |
| Blast Injuries | Deep cuts and lacerations from shrapnel. |
| Eye Injuries | Corneal burns and chemical injuries, potentially leading to blindness. |
| Head and Traumatic Brain Injuries | Injuries from falls due to explosions, including concussions and skull fractures. |
You should never use a battery that shows signs of swelling, leakage, or physical damage. Dispose of damaged batteries properly to avoid battery failure and personal injury.
Manufacturing Defects
Manufacturing defects, although less common, remain a significant cause of battery failure. Poor manufacturing practices, flawed assembly, or contamination during production can lead to internal short-circuits and thermal runaway. You may encounter functional failures, such as open-circuit or short-circuit issues, or subtle defects that only appear under specific conditions.
- Functional Failures: Open-circuit or short-circuit failures during use.
- Subtle Defects: Issues that manifest under certain conditions, leading to safety problems.
Improper design and lack of quality control can result in catastrophic battery failure, including fire and explosion. Internal short-circuits from manufacturing defects are a major cause of lithium battery explosions. You should always purchase batteries from reputable manufacturers to reduce the risk of battery failure.
Common manufacturing defects include:
- Capacity degradation
- Internal short-circuits
- Thermal runaway
External Heat Sources
External heat sources, such as fire or direct sunlight, can trigger battery failure and explosions. As the battery temperature rises, flammable electrolytes break down, producing gases that increase internal pressure. Exposure to excessive heat accelerates internal reactions, raising the risk of thermal runaway and short-circuit.
- Leaving devices in hot environments, like cars or under direct sunlight, can initiate thermal runaway.
- High temperatures from external sources elevate internal battery temperatures beyond safe limits, triggering battery failure.
You should always store batteries in cool, dry places and avoid exposing them to fire or direct sunlight. Proper handling reduces the risk of battery failure and explosion.
⚠️ Note: Continued use of damaged batteries or exposure to heat sources significantly increases the risk of battery failure and explosion. Always inspect your devices for signs of damage and avoid unsafe environments.
How Does the Li-ion Battery Catch Fire

Chain Reaction Process
You need to understand the chain reaction process that leads to a lithium-ion battery catching fire. When the battery heats up, a series of chemical and physical events occur. Each step increases the risk of fire and explosion. Here is the sequence:
- Solid Electrolyte Interphase (SEI) layer decomposes above 80°C, exposing the anode to the electrolyte.
- Anode-electrolyte reactions begin at around 120°C, releasing heat and gases.
- Separator melts or breaks down at 130°C, causing internal short circuits.
- Electrolyte decomposes between 190°C and 200°C, generating more heat and flammable gases.
- Cathode decomposes as temperatures rise, releasing oxygen that fuels combustion.
- Electrochemical energy releases uncontrollably, adding to heat generation.
- Lithiation of the cathode occurs, causing further exothermic reactions.
- Open circuit voltage drops due to lithiation and short circuits.
Each stage pushes the battery closer to a dangerous fire event. You should recognize that these reactions happen quickly once the battery reaches critical temperatures.
Gas Formation and Pressure
During thermal runaway, you see gas formation inside the battery. These gases increase internal pressure and raise the risk of fire. The table below shows how this process unfolds:
| Evidence Description | Details |
|---|---|
| Gas Formation Mechanism | Exothermic reactions generate flammable gases, increasing pressure. |
| Explosion Risk | Gas buildup can rupture the cell and ignite, causing fire and explosion. |
| Types of Gases | Hydrogen, carbon monoxide, carbon dioxide, methane, and propane. |
You should know that the buildup of gases can rupture the battery shell. When the shell breaks, flammable gases escape and ignite, leading to a fire. This process often happens in seconds, giving you little time to react.
⚠️ If you notice swelling or hissing sounds from your device, stop using it immediately. These signs indicate gas buildup and a high risk of fire.
Ignition and Explosion
The ignition point varies depending on the battery chemistry. Once the temperature reaches the ignition point, the battery can catch fire or explode. The table below lists common lithium-ion battery chemistries and their ignition points:
| Chemistry | Abbreviation | Ignition Point (°C) |
|---|---|---|
| Lithium Iron Phosphate | LiFePO4 | 270 |
| Lithium Nickel Manganese Cobalt Oxide | LiNiMnCoO2 | 210 |
| Lithium Nickel Cobalt Aluminum Oxide | LiNiCoAlO2 | 150 |
| Lithium Manganese Oxide | LiMn2O4 | 250 |
| Lithium Cobalt Oxide | LiCoO2 | 150 |
| Lithium Titanate Oxide | Li2TiO3 | 177 |
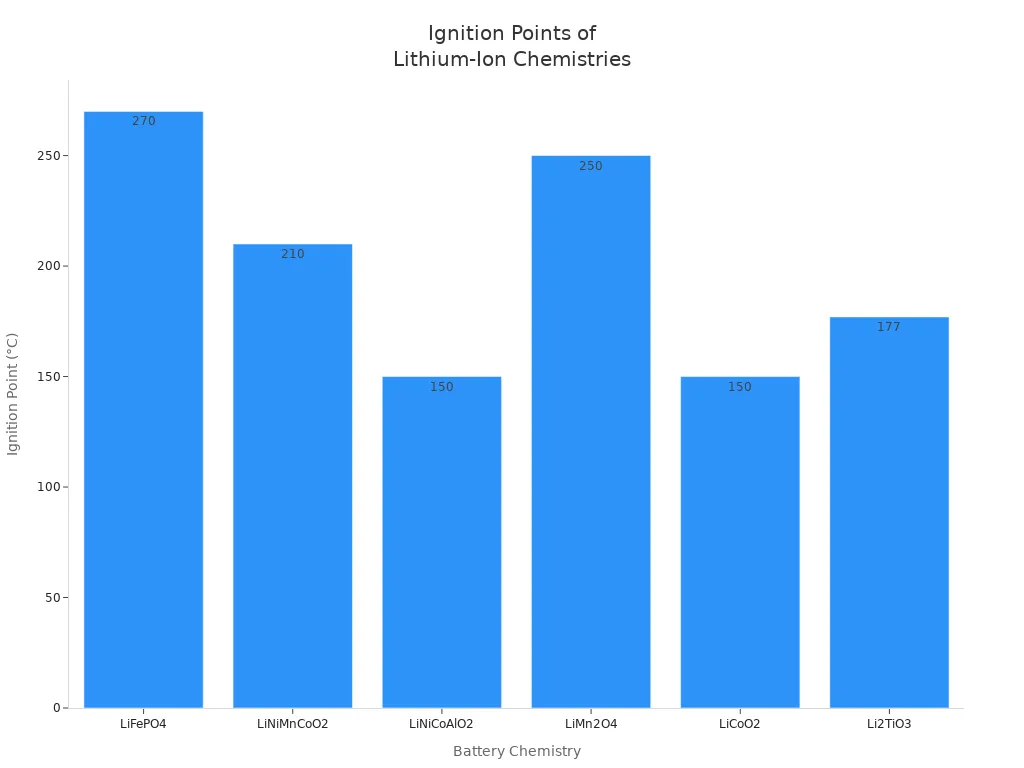
You should remember that once the battery reaches its ignition point, a fire can start instantly. The combination of high temperature, flammable gases, and oxygen creates an environment where fire spreads rapidly. You must handle batteries with care and avoid exposing them to heat sources.
Risk Factors for Lithium-Ion Battery Explosions
Environmental Conditions
You face increased risk of lithium-ion battery explosions when environmental conditions become unfavorable. Elevated temperatures, high humidity, and water ingress all contribute to battery instability.
- Elevated temperatures accelerate chemical reactions inside the battery, raising the chance of thermal runaway.
- High humidity can cause moisture to penetrate the battery casing, leading to internal corrosion and short circuits.
- Water ingress, especially saltwater, poses a severe hazard. After Hurricane Ian in 2022, several electric vehicle batteries submerged in saltwater ignited, demonstrating how water exposure and heat can trigger fires.
You should always store your lithium-ion battery devices in cool, dry places. Avoid leaving them in vehicles during hot weather or exposing them to rain and flooding.
User Handling
Improper handling by users remains a leading cause of lithium-ion battery explosions.
- Using incompatible chargers can result in overheating or damage, posing a safety hazard.
- Reading the device’s user manual helps you select the correct battery type and charger.
- Always use chargers specifically designed and approved by the manufacturer for your device.
- Third-party or incompatible chargers may cause overcharging, overheating, or short circuits, increasing the risk of battery failure or fire.
- Using the manufacturer’s charger reduces the chance of lithium-ion battery fires and explosions.
You should inspect your devices regularly for signs of damage or swelling. Handle batteries gently and avoid dropping or puncturing them.
Battery Design
Battery design plays a crucial role in reducing explosion risk. Researchers at Texas A&M University developed a new anode architecture that prevents lithium from accumulating outside the anode, which can cause unintended contact and explosions. This innovation also allows for faster charging and safer operation. Manufacturers incorporate several safety features into lithium-ion battery cells:
| Feature | Description |
|---|---|
| Current Interrupt Device (CID) | Disconnects the circuit when internal pressure exceeds safe limits, preventing thermal runaway. |
| Safety Vent | Releases gases in a controlled manner if pressure rises after CID activation, preventing explosions. |
| Notched Can Bottom | Serves as a backup venting pathway if the top vent fails, providing additional protection. |
| Center Pin | Guides gas release during venting, preventing uncontrolled rupture. |
The new carbon nanotube design for the anode allows for safe storage of lithium ions, reducing fire risk. You benefit from these advancements when you choose devices from reputable brands that prioritize battery safety.
Prevention and Safety Tips
Safe Charging
You can greatly reduce the risk of fire hazard by following safe charging practices for lithium-ion batteries. Always charge batteries at a slow rate to minimize stress on the cells. Disconnect the charger once the battery reaches full capacity to avoid overcharging. Use a charger rated for about one-quarter of the battery’s capacity. This approach ensures safe charging and extends battery life. Charging up to 80% instead of 100% also helps reduce wear and supports battery safety.
⚡ Tip: Only use chargers recommended by the manufacturer to maintain safety protection and prevent improper usage.
Proper Storage
Proper storage of lithium-ion batteries plays a key role in battery safety. Store batteries in cool, shaded areas to maintain performance and stability. Isolate batteries in fire-rated buildings to protect property and assist fire crews in emergencies. Climate control and fire suppression systems help mitigate volatility and prevent thermal events. Use up-to-date sensors and detectors to prevent off-gassing or thermal runaway.
A temperature-controlled environment, ideally around 15°C (59°F), keeps batteries stable. Store batteries at about 40% state of charge and ensure voltage stays between 2.0 and 4.1 volts.
- Avoid overcharging during storage.
- Never store batteries in hot or humid places.
Recognizing Warning Signs
You should always watch for warning signs that indicate a lithium-ion battery may fail.
- Heat: If the battery feels extremely hot, stop using it.
- Swelling: Look for lumps, bulges, or leakage.
- Noise: Hissing or crackling sounds signal distress.
- Odor: A strong or unusual smell means trouble.
- Smoke: Visible smoke requires immediate action.
? Alert: Remove the device from use and seek professional help if you notice any of these signs. Quick action provides essential safety protection.
Quality Products
Choosing quality products with proper certifications ensures battery safety. Look for lithium-ion batteries with UL certification, which involves rigorous testing for electrical, mechanical, and environmental safety. Regular inspections by UL help manufacturers maintain high standards, reducing risks of overheating and fires.
Poor-quality materials or improper assembly increase the risk of overheating, electrolyte leakage, and explosions. Proper design and adherence to safety standards minimize these risks.
- Always select batteries and devices from reputable brands.
- Check for UL Listing or similar certifications before purchase.
✅ Note: Certified products offer better safety protection and reliability for your devices.
Lithium-ion batteries explode mainly because of thermal runaway, internal short circuits, and flammable electrolytes. You can minimize risks by following proven safety measures:
| Dangers of Lithium Batteries | Prevention Measures |
|---|---|
| Explosion | Use protective cases and avoid extreme temperatures. |
| Fire | Store batteries safely and use proper chargers. |
| Thermal Runaway | Inspect batteries and dispose of damaged ones. |
Public awareness and regulatory compliance play a vital role. You should prioritize battery safety, choose certified products, and stay informed about best practices. Your actions protect both your devices and your environment.
-

 May.2026.02.27Lithium-Ion Batteries: The Six Constraints Blocking the Path to PerfectionLearn More
May.2026.02.27Lithium-Ion Batteries: The Six Constraints Blocking the Path to PerfectionLearn More -

 May.2026.02.25Li-Polymer Battery 5000mAh: Complete Technical & OEM GuideLearn More
May.2026.02.25Li-Polymer Battery 5000mAh: Complete Technical & OEM GuideLearn More -

 May.2026.02.24The Unparalleled Advantages of Lithium-Ion Batteries Over Traditional BatteriesLearn More
May.2026.02.24The Unparalleled Advantages of Lithium-Ion Batteries Over Traditional BatteriesLearn More -

 May.2026.02.243.6 Volt Battery: Complete Technical Guide for Engineers & BuyersLearn More
May.2026.02.243.6 Volt Battery: Complete Technical Guide for Engineers & BuyersLearn More -

 May.2026.02.24What Is a 3.8V LiPo Battery? A Complete Engineering & OEM GuideLearn More
May.2026.02.24What Is a 3.8V LiPo Battery? A Complete Engineering & OEM GuideLearn More















