Custom Li-ion Battery Design for Medical Devices (2025 Comprehensive Guide)
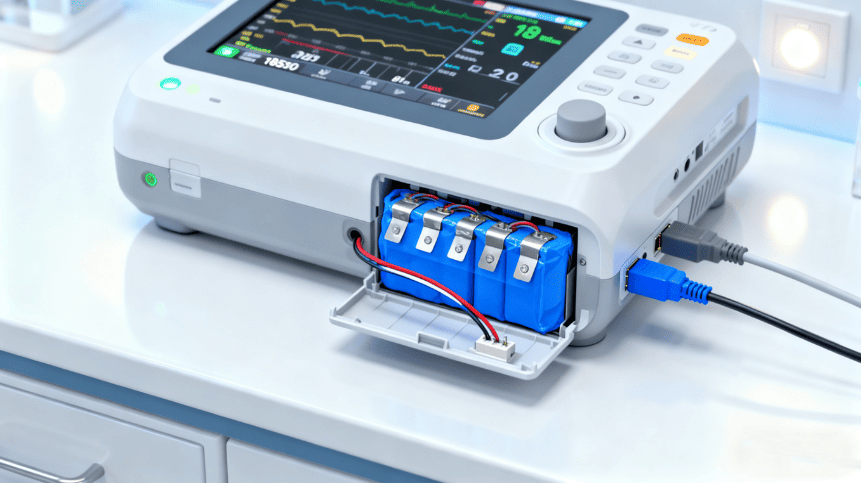
Medical devices demand a level of precision, reliability, and safety that surpasses almost every other industry. Whether it’s an insulin pump, portable ECG monitor, surgical tool, infusion pump, wearable diagnostic sensor, or hearing aid, every medical device relies on one thing in common: a safe and highly reliable lithium-ion battery.
In 2025, custom Li-ion battery design has become one of the fastest-growing technical segments in the medical engineering field. According to MarketsandMarkets, the global medical battery market is projected to reach USD 4.2 billion by 2027, growing at 6.2% CAGR, largely driven by miniaturization, longer runtimes, and the rising adoption of home-care technologies.
This guide explains everything you need to know before designing or sourcing a custom Li-ion battery pack for medical devices—with real data, engineering considerations, safety testing, and manufacturing best practices.
1. Why Li-ion Batteries Dominate Medical Devices in 2025
1.1 Energy Density Advantage
Li-ion batteries offer 2–3× higher energy density than NiMH or alkaline, enabling smaller device footprints.
| Battery Chemistry | Gravimetric Energy Density (Wh/kg) | Typical Medical Use |
|---|---|---|
| Li-ion (NMC) | 180–250 | Wearables, portable monitors |
| Li-ion (LCO) | 150–200 | Imaging, diagnostic |
| LiFePO4 (LFP) | 120–160 | Surgical tools, emergency equipment |
| NiMH | 60–120 | Legacy devices |
| Alkaline | 80–110 | Disposable medical tools |
Source: Battery University 2024, IEA Energy Storage Report 2025
1.2 Low Self-Discharge, Critical for Hospital Environments
Li-ion cells maintain charge far longer during storage:
-
Li-ion: 1.5–2.0% per month
-
NiMH: 15–25% per month
For devices stored for long periods (AEDs, emergency kits), this is essential.
1.3 Support for Continuous Monitoring Devices
Medical OEMs increasingly rely on Li-ion for:
-
24/7 patient monitoring
-
Wireless data transmission
-
Wearable medical IoT devices
-
Home-care equipment replacing hospital-grade hardware
2. Key Considerations When Designing Custom Li-ion Medical Batteries
2.1 Cell Chemistry Selection
Different medical devices require specific chemistry properties.
| Chemistry | Advantages | Best for |
|---|---|---|
| NMC | High energy density, stable performance | Wearable monitors, handheld tools |
| LCO | High voltage, low impedance | Imaging devices, high-drain |
| LFP | Exceptional safety, long cycle life | Surgical tools, home-care machines |
| NCA | Higher energy than NMC | Mobility devices (power chairs) |
Recommendation:
For most portable medical devices, NMC or LFP provide the best balance of safety and energy.
2.2 Form Factor Strategy (Custom vs Off-the-Shelf)
Custom cells allow:
-
Unique shapes (curved, ultra-thin, flexible)
-
Higher volumetric efficiency
-
Perfect fit for compact housings
A&S Power commonly designs custom forms for:
-
Smart insulin pumps
-
Wearable ECG/EEG
-
Smart thermometers
-
Diagnostic patches
2.3 Battery Management System (BMS) Requirements
A medical-grade BMS must include:
-
Over-charge protection
-
Over-discharge protection
-
Over-current + short-circuit protection
-
Cell balancing
-
Temperature monitoring
-
Redundant safety layers
-
Optional CAN/UART communication
In FDA-regulated devices, redundancy is essential (dual temperature sensors, dual MOS cutoff, etc.)
3. Medical Safety Certification Requirements
Medical Li-ion batteries must comply with specific global standards:
| Certification | Purpose | Required Region |
|---|---|---|
| UN38.3 | Transport safety | Global |
| IEC62133-2 | Rechargeable Li-ion safety | EU/Global |
| UL2054 | Household/commercial Li-ion | US |
| UL1642 | Cell-level safety | US |
| ISO 13485 | Medical device quality system | Global |
| FDA 510(k) | Device-level approval | US |
For 2025, IEC62133-2 and UN38.3 remain mandatory for nearly all medical products.
4. Runtime & Cycle Life Optimization (Engineering Focus)
4.1 Proper Capacity Selection (Right-Sizing)
Over-sizing increases cost and footprint; under-sizing causes failure.
Formula for calculating required capacity:
Battery Capacity (mAh) = (Device Current Draw mA × Usage Hours) / System Efficiency %
4.2 Expected Cycle Life
-
NMC: 500–800 cycles
-
LFP: 2000–3500 cycles
-
LCO: 400–600 cycles
For long-term diagnostic tools, LFP provides the best durability.
5. Environmental & Reliability Testing (2025 standards)
Medical batteries must survive:
-
Drop tests
-
Vibration
-
Temperature cycling
-
Humidity resistance
-
High-altitude storage
-
Accelerated aging
-
Electrostatic discharge (ESD)
A&S Power performs additional tests such as:
-
10× charge/discharge simulation at extreme temperatures
-
Salt spray for hospital disinfection environments

6. Why Medical OEMs Choose A&S Power for Custom Li-ion Batteries
A&S Power specializes in:
About A&S Power
A&S Power is a leading manufacturer of Lithium-ion and Lithium Polymer batteries for medical, wearable, industrial, consumer electronics, and IoT devices. With 15+ years of experience, full certification capabilities, and advanced custom engineering, A&S Power supports global OEMs in building safer and more reliable energy systems.
-

 May.2026.02.27Lithium-Ion Batteries: The Six Constraints Blocking the Path to PerfectionLearn More
May.2026.02.27Lithium-Ion Batteries: The Six Constraints Blocking the Path to PerfectionLearn More -

 May.2026.02.25Li-Polymer Battery 5000mAh: Complete Technical & OEM GuideLearn More
May.2026.02.25Li-Polymer Battery 5000mAh: Complete Technical & OEM GuideLearn More -

 May.2026.02.24The Unparalleled Advantages of Lithium-Ion Batteries Over Traditional BatteriesLearn More
May.2026.02.24The Unparalleled Advantages of Lithium-Ion Batteries Over Traditional BatteriesLearn More -

 May.2026.02.243.6 Volt Battery: Complete Technical Guide for Engineers & BuyersLearn More
May.2026.02.243.6 Volt Battery: Complete Technical Guide for Engineers & BuyersLearn More -

 May.2026.02.24What Is a 3.8V LiPo Battery? A Complete Engineering & OEM GuideLearn More
May.2026.02.24What Is a 3.8V LiPo Battery? A Complete Engineering & OEM GuideLearn More















