Stanford technology predicts the slow death of lithium-ion batteries
Stanford technology leads progress in lithium-ion battery research. You see urgent challenges in modern industries, including safety, efficiency, and sustainability. Recent studies point out key concerns:
- Spent lithium-ion batteries create environmental and safety risks.
- Recycling methods waste energy and valuable lithium.
- Electrolyte contamination threatens recovery and sustainability.
Stanford drives innovation that addresses these problems. You benefit from academic research that shapes the technology you use every day.
Key Takeaways
- Stanford's research leads to safer lithium-ion batteries through innovations like polymer-based electrolytes and solid-state barriers.
- Recycling lithium-ion batteries significantly reduces greenhouse gas emissions, water use, and energy consumption, making it an essential practice for sustainability.
- New charging methods developed at Stanford can extend battery lifespan by up to 50%, enhancing efficiency and performance for everyday devices.
- Sodium-ion batteries present a promising alternative to lithium-ion, offering lower costs and sustainability, but face challenges in energy density and maturity.
- Stanford's collaboration with industry partners accelerates the development of advanced battery technologies, benefiting consumers with improved performance and reliability.
Stanford technology in batteries

Research leadership
You see Stanford technology at the forefront of battery innovation. The university stands out for its commitment to solving real-world problems in energy storage. Researchers at Stanford have made several breakthroughs that set them apart from other institutions:
- They developed a new coating for lithium metal batteries, which improves safety and extends battery life. This advancement is crucial for electric vehicles.
- Artificial intelligence, applied by Stanford-led teams, has reduced battery testing times. You benefit from faster development of efficient batteries.
- Scientists at Stanford created a novel lithium-based electrolyte. This discovery could transform battery technology for electric vehicles.
- A new class of solid materials, discovered at Stanford, may replace hazardous liquid electrolytes in lithium-ion batteries.
- Researchers enhanced battery efficiency and safety by adding polymers and fireproofing to battery components.
Stanford technology also includes a patented polymerization strategy for silicon-based anodes. This innovation addresses the expansion and cracking issues in traditional silicon anodes, allowing batteries to last through 5,000 charge cycles. Another achievement is a real-time health monitoring system for lithium-ion batteries. This system improves the accuracy of monitoring key variables, which helps ensure battery longevity and safety.
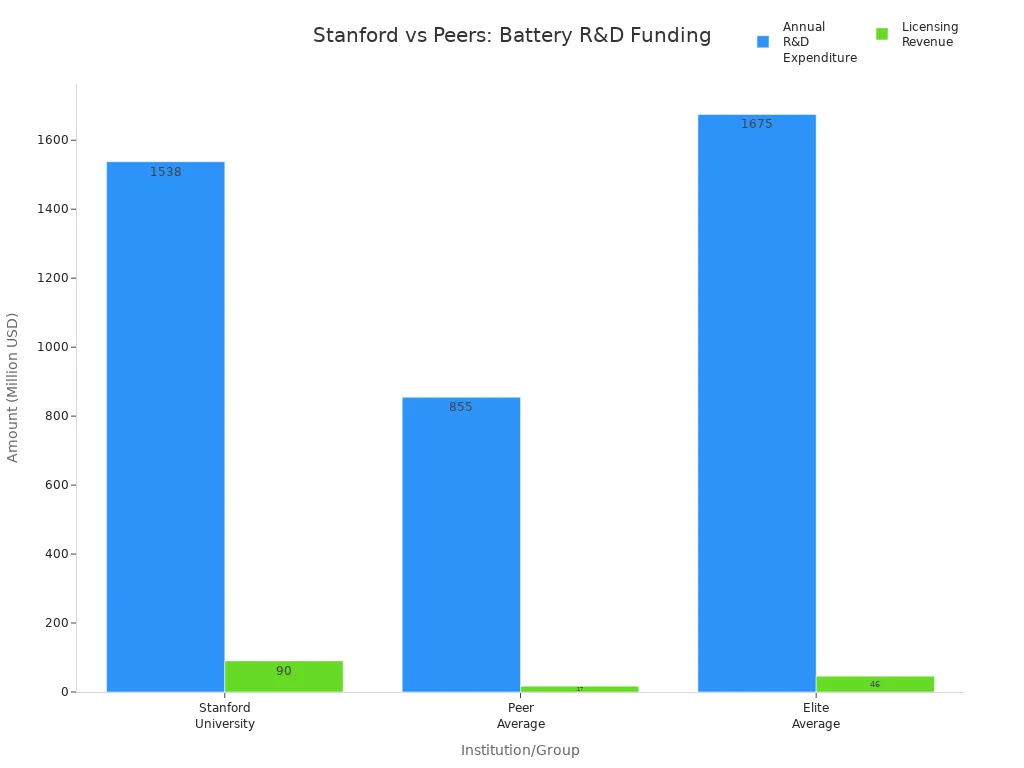
Note: Stanford’s research funding and resources surpass many peer institutions. The university operates multiple labs focused on materials science and battery development. Strong partnerships with industry leaders like Tesla and Panasonic further boost its capabilities.
| Category | Stanford University | Peer Average | Elite Average |
|---|---|---|---|
| Annual R&D Expenditure (FY2023) | $1,538M | $855M | $1,675M |
| Licensing Revenue | $90M | $17M | $46M |
Industry collaboration
You benefit from Stanford technology because the university works closely with industry partners. These collaborations speed up the development of better batteries. For example:
- Machine learning advancements from industry partnerships help create more durable batteries for fast-charging electric vehicles.
- Computer vision tools reveal new insights into how lithium-ion batteries work.
- Joint projects allow researchers to analyze X-ray movies and understand how nanoparticles behave inside battery electrodes.
Stanford technology bridges academic research and industry needs. You see real progress in battery performance and safety because of these efforts.
Current batteries: challenges and limits
Safety and efficiency
You rely on batteries every day, but current technology faces serious safety and efficiency challenges. As energy density increases, so do the risks. Thermal runaway can cause fires or explosions, which makes many people worry about using these devices. Overcharging, deep discharging, and mechanical wear can damage battery cells. This damage leads to performance issues and shortens the lifespan of your devices.
Manufacturing defects and material impurities also create hazards. Even small contaminants can compromise battery integrity. Poor maintenance, such as exposing batteries to extreme temperatures, increases the risk of failure. You need to know that rigorous testing is essential to ensure reliability and performance.
| Cause | Description |
|---|---|
| Thermal runaway | Overheating can lead to fires or explosions. |
| Improper usage | Overcharging or deep discharging can cause catastrophic outcomes. |
| Manufacturing defects | Internal short circuits may result from separator failures. |
| Material impurities | Contaminants increase the risk of battery failure. |
| Poor maintenance | Extreme temperatures raise the chance of hazardous incidents. |
You see that traditional lithium-ion batteries also suffer from stress in electrodes during charging and discharging. This stress destabilizes active materials and reduces efficiency over time.
Environmental impact
You may not realize the environmental cost of producing and disposing of lithium batteries. The process uses large amounts of water and releases significant carbon emissions. For example, producing one tonne of lithium requires about 2 million tonnes of water. In Chile, 65% of the region’s water goes to lithium extraction. Almost 4 tonnes of CO2 are released during the production of a single electric car.
| Evidence Type | Statistic/Fact |
|---|---|
| Carbon Emissions | 46% of EV carbon emissions come from production, compared to 26% for ICE vehicles. |
| CO2 Emissions per Electric Car | Almost 4 tonnes of CO2 are released during the production of a single electric car. |
| Water Usage for Lithium Production | Producing one tonne of lithium requires approximately 2 million tonnes of water. |
| Water Usage in Chile | 65% of the region’s water was used for lithium extraction in Chile. |
| CO2 Emissions from Lithium Mining | Lithium mining emits 15 kg of CO2 per kg of lithium produced. |
| Environmental Pollution | Improperly disposed batteries leach chemicals into soil and water, contaminating ecosystems. |
Improper disposal of batteries causes soil and water pollution. Toxic substances like lithium and cobalt can leak into the environment. Heavy metals may enter groundwater if batteries end up in landfills. Fires at landfill sites can release harmful fumes. Studies show that over 98% of lithium-ion batteries end up in landfills, increasing the risk of environmental contamination.
Stanford breakthroughs
Polymer-based electrolyte
You can see how Stanford researchers have transformed battery safety and performance with their polymer-based electrolyte technology. Traditional electrolytes in batteries often suffer from low conductivity, poor stability, and high flammability. Stanford's new polymer-based electrolytes address these issues directly.
| Feature | Conventional Electrolytes | Stanford's Polymer-based Electrolytes |
|---|---|---|
| Ionic Conductivity | Low | Enhanced by three orders of magnitude |
| Electrochemical Stability | Poor | Improved |
| Mechanical Strength | Deteriorated | Enhanced |
| Flammability | High | Low (tolerates flame for 3 seconds) |
You benefit from the FDMB electrolyte, which resists ignition even when exposed to direct flame for at least three seconds. In contrast, conventional carbonate electrolytes ignite instantly. This improvement means you can expect safer batteries in your devices and vehicles.
Stanford's research also introduces a design principle that helps scientists design a better battery. This principle opens the door to new electrolyte innovations, making it easier for manufacturers to create better batteries for a wide range of uses.
Engineers at the Zhenan Bao Lab have developed an elastic lithium-ion conductor with dual covalent and dynamic hydrogen bonding crosslinks. This design provides high mechanical resilience and maintains excellent ionic conductivity. You can expect this technology to scale up for commercial battery manufacturing, making safer and more efficient batteries widely available.
Solid-state barriers
You may have heard about solid-state electrolyte batteries as a safer alternative to traditional lithium-ion batteries. Stanford's breakthroughs in solid-state technology set a new standard for safety and performance. Researchers created a new class of solid electrolytes using lithium, boron, and sulfur. These materials replace flammable liquid electrolytes, reducing the risk of combustion.
Stanford's solid-state electrolytes are about twice as stable as leading alternatives. You gain peace of mind knowing that these batteries maintain conductivity even as they decompose. This means your devices and vehicles can function longer and more safely. The innovation could double the energy density of lithium-ion batteries, giving electric vehicles longer driving ranges and making solid-state electrolyte batteries a practical choice for the future.
A recent study from Stanford highlights that these solid-state materials not only improve safety but also support higher energy storage. You see the benefits in both everyday electronics and advanced transportation.
Factory charging method
Stanford's factory charging method changes how you think about battery lifespan and efficiency. Traditional charging methods often waste lithium during the first charge, which shortens battery life. Stanford researchers discovered that charging batteries at high currents during the initial cycle creates a protective SEI (solid electrolyte interphase) layer. This layer helps preserve lithium and extends the battery's lifespan.
| Evidence Description | Impact on Battery Lifespan and Efficiency |
|---|---|
| High currents during initial charging deplete lithium supply | Prolongs battery life by creating a protective SEI layer |
| Initial lithium loss during first charge | Minimizes overall lithium loss over time |
| Charging at high currents increases lifespan by 50% | Enhances cycling efficiency and performance |
| Deactivation of 30% lithium upfront vs. 9% with previous methods | Creates headspace in electrodes for better cycling efficiency |
You benefit from this method because it increases battery lifespan by up to 50%. The process also improves cycling efficiency, so your devices last longer between charges. Manufacturers can use this approach to produce batteries that perform better and last longer, making solid-state electrolyte batteries even more reliable.
New battery design
Stanford's new battery design focuses on real-world performance. Researchers at the SLAC Battery Center tested 92 commercial lithium-ion batteries over two years. They used discharge profiles that mimic actual driving behaviors, such as stop-and-go traffic and periods of inactivity.
- Real-world driving conditions can extend the lifespan of electric vehicle batteries by up to 40% compared to previous estimates.
- Batteries tested under dynamic discharge patterns experienced slower degradation rates than those tested under traditional lab conditions.
- The study shows that your electric vehicle battery can last longer if it faces varied usage, not just constant high demand.
You see the impact of this research in the next generation of batteries. Stanford's approach helps manufacturers design batteries that perform better in real-world conditions. This means you get more reliable and longer-lasting batteries for your devices and vehicles.
By focusing on polymer-based electrolytes, solid-state barriers, innovative charging methods, and new battery designs, Stanford continues to lead the way in battery technology. You benefit from safer, more efficient, and longer-lasting batteries that support a sustainable future.
Battery recycling at Stanford
Reducing environmental impact
You play a key role in shaping a cleaner future by supporting battery recycling. Stanford leads the way with advanced recycling methods that lower the environmental footprint of used batteries. When you recycle, you help reduce greenhouse gas emissions, water use, and energy consumption. A lifecycle analysis from Stanford shows that recycling lithium-ion batteries emits less than half the greenhouse gases compared to mining new materials. The process also uses about one-fourth the water and energy.
| Environmental Impact | Reduction Percentage |
|---|---|
| Greenhouse Gas Emissions | 58% to 81% less |
| Water Usage | 72% to 88% less |
| Energy Consumption | 77% to 89% less |
You can see that recycling makes a real difference. By choosing recycled materials, you help protect water resources and reduce pollution. Stanford’s research highlights that these efforts keep toxic metals out of landfills and prevent harmful chemicals from entering the soil and water.
Strengthening supply chains
You benefit from stronger supply chains when you support battery recycling. Stanford’s programs use new technologies to recover valuable materials like lithium and cobalt. These innovations include AI-driven sorting and automated disassembly, which boost recovery rates to over 95%. You help reduce the need for mining and cut transport distances from 35,000 miles for mining to just 140 miles for recycling.
- Recycling lithium-ion batteries can reduce greenhouse gas emissions by up to 81%.
- Water use drops by as much as 88%.
- Energy consumption falls by up to 89% compared to mining.
- Reductive calcination and advanced sorting improve recovery rates and lower costs.
- Emissions decrease by up to 74% with new recycling techniques.
Stanford’s research projects that by 2035, recycling could reuse over 4 million tons of end-of-life batteries, saving billions of dollars and securing critical materials for future use. You help create a more resilient and sustainable supply chain every time you recycle.
Beyond lithium: sodium-ion batteries

Alternative solutions
You may wonder if there are alternatives to lithium for energy storage. Sodium-ion batteries have gained attention as a promising option. Sodium is much more abundant than lithium, which means you can find it almost everywhere. This abundance could help lower costs and make battery production more sustainable. The chemistry of sodium-ion batteries is similar to lithium-ion, so manufacturers can use existing processes to produce them. You might see these batteries used in large-scale energy storage, such as for the power grid.
Here is a comparison of the main advantages and limitations:
| Advantages of Sodium-Ion Batteries | Limitations of Sodium-Ion Batteries |
|---|---|
| Sodium is more abundant and widely available than lithium. | They have lower energy density compared to lithium-ion batteries. |
| Lower cost of sodium could lead to more affordable battery production at large scale manufacturing. | It is not as mature as lithium-ion technology. |
| Chemistry of sodium-ion is similar to lithium-ion, allowing the use of existing manufacturing processes. | Some sodium-ion battery chemistries may exhibit low life cycle and lower cycling stability. |
| Can be used in grid scale energy storage applications. | Performance depends on specific materials used for electrodes and electrolytes. |
| Might have reduced environmental impact during disposal/recycling. | Potential safety advantages need further testing to ensure safety standards are met. |
You see that sodium-ion batteries offer several benefits, but they also face important challenges. Lower energy density means they store less power for the same size, which can limit their use in portable electronics or electric vehicles.
Future prospects
You may ask what the future holds for sodium-ion technology. Stanford researchers point out that several hurdles remain before these batteries can compete with lithium-based options. The technology is still in its early stages, and the supply chain for materials is not well established. Only a few companies currently produce sodium-ion batteries, which keeps costs high. The shape and flexibility of these batteries are also limited, and their cycle life does not yet match that of lithium iron phosphate batteries.
You can see the main challenges ahead:
- Significant technological advancements are needed for sodium-ion batteries to compete with lithium-ion.
- Increasing energy densities and reducing reliance on critical minerals will help improve competitiveness.
- The timeline for reaching price parity with lithium-ion batteries remains uncertain.
- Market changes and supply chain disruptions could affect the adoption of sodium-ion technology.
Tip: You can expect sodium-ion batteries to play a bigger role in grid storage and backup power, especially where cost and sustainability matter more than size or weight.
You will likely see more research and development in this area. As technology improves, sodium-ion batteries could become a key part of the energy landscape.
Real-world impact
Electric vehicles
You see the benefits of Stanford’s battery research every time you drive or ride in an electric vehicle. Stanford scientists have worked with industry partners to collect and analyze real driving data. For example, Volkswagen shared 3,750 hours of battery management system data from an Audi e-tron SUV driven in the San Francisco Bay Area. Researchers measured changes in current and voltage during 529 acceleration events and 392 braking events over a year. They found that battery resistance decreased in cooler months and increased in warmer months. This means your battery may perform better in certain seasons.
| Measurement Focus | Details |
|---|---|
| Resistance During Driving | Measured during 529 acceleration and 392 braking events |
| Seasonal Battery Health | Resistance decreased in cooler months, increased in warmer months |
| Energy and Power Assessment | Calculated using year-long data from Audi e-tron |
| Data Source | 3,750 hours of BMS data from Audi e-tron in San Francisco Bay Area |
You benefit from these findings because they help engineers design batteries that last longer and perform better in real-world conditions. This research supports the growth of electric vehicles by making them more reliable and efficient.
Consumer electronics
You rely on your phone, laptop, and wearable devices every day. Stanford’s battery innovations have made these devices safer, longer-lasting, and more powerful. New battery designs now achieve energy densities above 400 Wh/kg, which means your devices can run longer between charges. These batteries also last for more than 500 cycles, so you do not need to replace them as often.
| Feature | Description |
|---|---|
| Energy Density | Over 400 Wh/kg, allowing longer device usage |
| Cycle Life | More than 500 cycles, increasing battery longevity |
| Safety | No dendrite growth or gassing, making batteries safer |
| Manufacturing | Uses existing facilities with minimal changes |
| Application | Powers smartphones, laptops, and wearables |
| User Experience | Stable voltage and reliable power supply for better performance |
You notice that your devices hold power longer and stay safe during use. These improvements come from Stanford’s focus on real-world needs and practical solutions.
The future of batteries
Ongoing research
You see Stanford researchers pushing the boundaries of battery science every day. Their teams focus on next-generation lithium-metal batteries, which promise higher energy storage and longer life. One major challenge with these batteries is dendrite formation. Dendrites are tiny, needle-like structures that can grow inside batteries and cause short circuits or failure. To tackle this, Stanford scientists developed a new mathematical model. This model helps predict and control dendrite growth, making batteries safer and more reliable.
Researchers also experiment with new electrolytes. These materials can slow or even stop dendrite formation, which means your future devices could last longer and work more safely. The research team uses this model to guide the design of better batteries, reducing the need for trial-and-error in the lab. You benefit from this approach because it speeds up the development of advanced batteries. The next step involves building real devices based on these findings and testing them in practical situations.
Note: Ongoing projects at Stanford aim to create batteries that store more energy, last longer, and operate safely in a wide range of conditions.
Commercialization
You will soon see these innovations move from the lab to the marketplace. However, several challenges remain before advanced batteries become widely available. Solid-state electrolyte batteries, for example, face issues with surface defects. These defects can affect performance, so manufacturers must use precise methods to produce reliable batteries. Understanding how these batteries behave under different physical conditions is also crucial for large-scale production.
Stanford researchers highlight the risks of relying only on lithium-ion batteries. Supply chain problems, especially with materials like graphite, can disrupt production and raise costs. To address this, you see new efforts to develop sodium-ion batteries. These alternatives use more abundant materials and help reduce economic and security risks.
The STEER program at Stanford works to diversify energy storage options. You will hear more about research into long-duration storage, hydrogen technologies, and ways to decarbonize industry. These efforts aim to create a future where you have access to safer, more sustainable, and affordable energy solutions.
You see how stanford technology shapes the future of batteries. Researchers create new solutions like water-based batteries and liquid organic hydrogen carriers, which promise safer and longer-lasting energy storage. These advancements help industries and consumers move toward clean energy. You can expect ongoing research to deliver even better batteries for electric vehicles and the power grid.
You play a part in a future where energy storage is safer, more efficient, and sustainable.
FAQ
What makes Stanford’s battery research unique?
You see Stanford lead with real-world testing, advanced materials, and strong industry partnerships.
Stanford’s focus on safety, efficiency, and sustainability sets its research apart from other universities.
How do Stanford’s breakthroughs improve battery safety?
Stanford’s polymer-based electrolytes and solid-state barriers reduce fire risks.
| Feature | Benefit |
|---|---|
| Non-flammable | Safer devices |
| Stable materials | Fewer failures |
Can you recycle lithium-ion batteries easily?
You can recycle batteries at many collection points. Stanford’s research helps make recycling more efficient and less harmful to the environment.
- Look for local recycling programs
- Never throw batteries in the trash
Will sodium-ion batteries replace lithium-ion batteries soon?
You may see sodium-ion batteries in grid storage first. They need more research before replacing lithium-ion batteries in cars or phones.
Sodium-ion batteries offer lower cost and better sustainability, but energy density remains a challenge.
How can you benefit from Stanford’s battery innovations?
You get safer, longer-lasting batteries in your devices and vehicles.
- Enjoy fewer replacements
- Experience improved performance
- Support a cleaner environment
-

 May.2026.02.27Lithium-Ion Batteries: The Six Constraints Blocking the Path to PerfectionLearn More
May.2026.02.27Lithium-Ion Batteries: The Six Constraints Blocking the Path to PerfectionLearn More -

 May.2026.02.25Li-Polymer Battery 5000mAh: Complete Technical & OEM GuideLearn More
May.2026.02.25Li-Polymer Battery 5000mAh: Complete Technical & OEM GuideLearn More -

 May.2026.02.24The Unparalleled Advantages of Lithium-Ion Batteries Over Traditional BatteriesLearn More
May.2026.02.24The Unparalleled Advantages of Lithium-Ion Batteries Over Traditional BatteriesLearn More -

 May.2026.02.243.6 Volt Battery: Complete Technical Guide for Engineers & BuyersLearn More
May.2026.02.243.6 Volt Battery: Complete Technical Guide for Engineers & BuyersLearn More -

 May.2026.02.24What Is a 3.8V LiPo Battery? A Complete Engineering & OEM GuideLearn More
May.2026.02.24What Is a 3.8V LiPo Battery? A Complete Engineering & OEM GuideLearn More