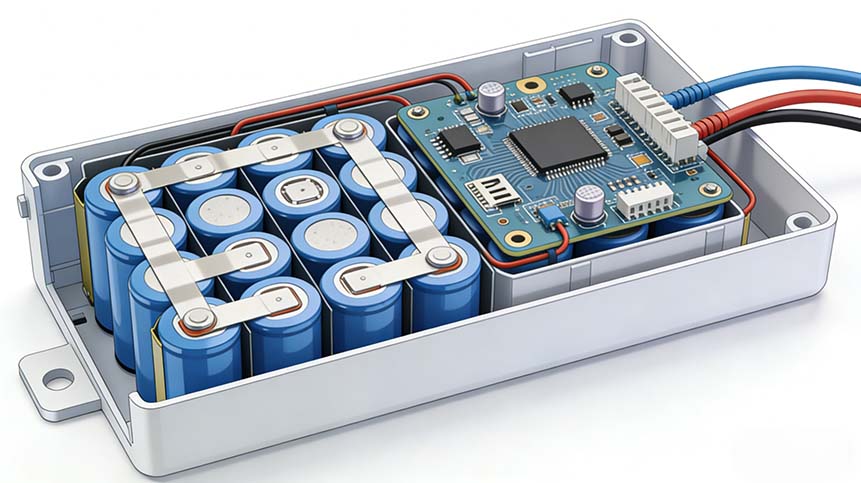
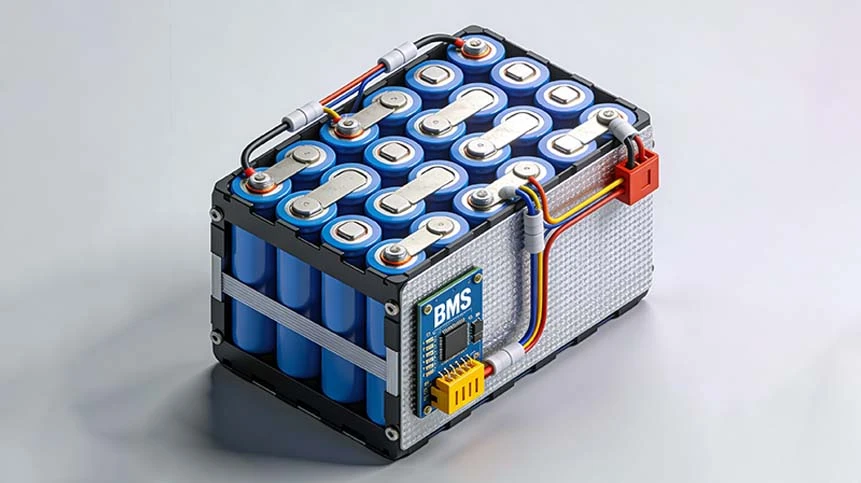
Direct answer: A custom medical device battery refers to any Li-ion or LiPo battery pack designed specifically for medical applications such as infusion pumps, portable ultrasound, patient monitoring, wearable biosensors, ventilators, CPAP devices, insulin pumps, surgical tools, and diagnostic instruments. These battery packs must comply with strict medical regulations such as IEC 62133, UN38.3, ISO 13485, IEC 60601-1, RoHS, REACH and require advanced BMS, long cycle life, high reliability, and medical-grade safety.
This guide provides an in-depth technical explanation covering aspects such as chemical material selection, battery pack architecture, safety design, medical certification, thermal management, reliability engineering, and manufacturing processes.
Table of Contents
- Overview of medical battery requirements
- Cell chemistry comparison (NCM, LCO, LFP, LiPo)
- Medical-grade safety requirements
- BMS for Medical Devices — functions, topology, protocols
- ISO13485 Medical Battery Design Workflow
- Cycle life engineering & longevity optimization
- Mechanical structure & thermal design
- Case studies: Insulin pump, patient monitor, portable ultrasound
- Testing protocols & certifications
- Failure mode analysis & risk control (FMEA)
- Supplier checklist for medical OEMs
1. Why Medical Device Batteries Require Custom Engineering
Unlike consumer electronics, medical batteries power life-critical systems. Battery reliability directly impacts patient safety and clinical continuity. Failures such as unexpected shutdowns, overheating, or inaccurate SOC readings are unacceptable in medical environments.
Key drivers for custom medical battery design:
- Need for consistent operation under tightly controlled conditions
- Battery must last the device’s lifetime (5–10+ years)
- Strict safety standards & certification requirements
- Reliable State-of-Charge (SOC) and State-of-Health (SOH) reporting
- Stable performance under storage, standby, and infrequent-use cycles
- Must work in emergency situations with zero failure tolerance
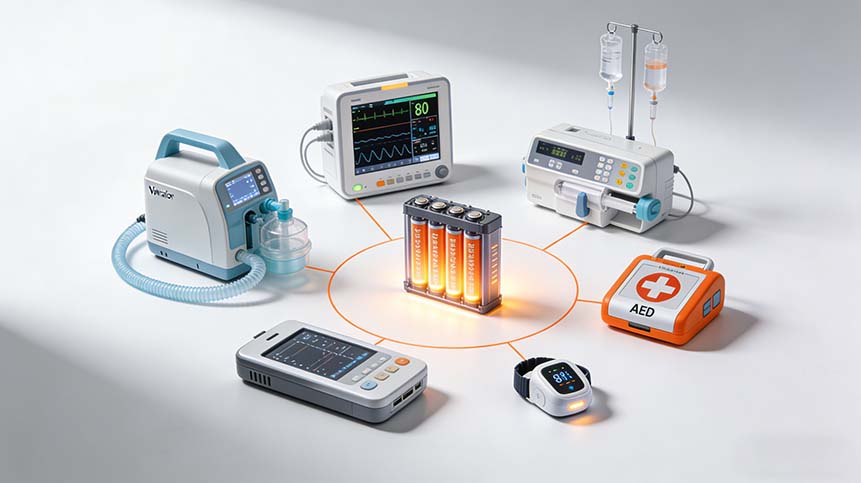
Common Medical Devices Using Custom Batteries
- Infusion / syringe pumps
- Portable patient monitor
- Electric breast pump
- Insulin pump
- Ventilator / CPAP
- Pulse oximeter
- Handheld diagnostic devices
- Wearable health monitors
2. Cell Chemistry Comparison for Medical Applications
Choosing the correct chemistry impacts safety, cycle life, stability, cost, and medical compliance.
| Chemistry | Nominal Voltage | Cycle Life | Safety | Energy Density | Medical Suitability |
|---|---|---|---|---|---|
| Lithium Cobalt Oxide (LCO) | 3.7V | 500–800 | Medium | High | For small high-density devices |
| Nickel Cobalt Manganese (NCM) | 3.6V | 700–1200 | High | Very High | Most medical devices |
| Lithium Iron Phosphate (LFP) | 3.2V | 2000–4000 | Very High | Medium | Ventilators, carts, large equipment |
| Lithium Polymer (LiPo) | 3.6V | 700–1500 | High | Very High | Wearables & portable devices |
Recommendation: NCM and LiPo are most commonly used due to high energy density, stable curves, and compatibility with compact medical equipment.
3. Medical Battery Safety Requirements
Medical batteries must pass multiple layers of compliance:
Required Standards
- IEC 62133 — lithium battery global safety standard
- UN38.3 — transport safety
- IEC 60601-1 — medical electrical equipment
- ISO 13485 — medical device quality management
- RoHS/REACH — environmental compliance
Key Safety Protections
- Overcharge / over-discharge
- Over-current & short-circuit
- Thermal protection & temperature sensors
- Cell balancing
- Fire-resistant casing (if required)
4. BMS Requirements for Medical Devices
A medical BMS must be smarter than normal Li-ion battery BMS.
Essential BMS Functions
- Accurate SOC & SOH estimation
- Redundancy for critical signals
- Active balancing optional for long-term accuracy
- Event logging for regulatory audits
- Communication: I2C, SMBus, UART, Bluetooth (wearables)
Advanced Functions
- Self-test diagnostics at startup
- Cold-weather charging protection
- Automatic derating based on temperature
- Real-time current consumption tracking
5. ISO 13485 Medical Battery Design Workflow
- Requirements definition with OEM
- Risk analysis (ISO 14971)
- Battery architecture selection
- Prototype fabrication
- Verification & validation testing
- IEC 62133 certification
- Pilot run & process validation
- Mass production & continuous improvement
6. Cycle Life Optimization
Medical devices require 3–8 years lifecycle. Cycle life can be tuned by:
- Limiting max charge to 4.15V
- Using high-cycle-life NCM or LFP chemistry
- Thermal control design
- Low C-rate charge/discharge strategies
7. Mechanical Structure & Thermal Design
Medical device housings are often compact, requiring customized structural engineering.
Design Considerations
- Fire-resistant ABS/PC enclosure
- Thermal pads for heat transfer
- Shock-absorption foam structures
- Medical-grade connectors
8. Case Studies
A. Insulin Pump Battery (LiPo 900–1200 mAh)
- Small, lightweight
- Stable discharge curve
- BMS with precision SOC algorithm
B. Portable Patient Monitor (Li-ion 7.4V 4000–6000 mAh)
- Medium-sized pack
- 2S2P or 2S3P configuration
- Strict temperature monitoring
C. Ultrasound Probe Battery (3.6V 3000–3500 mAh)
- High pulse power
- Premium high-discharge cells
9. Certification Testing Protocol
Standard tests include:
- Vibration
- Free-fall
- Charge retention
- Thermal stability
- Forced discharge
10. Failure Mode Control (FMEA)
Common failure modes:
- Swelling due to overcharge
- Capacity drop due to aging
- Connector corrosion
- Thermal imbalance
11. Supplier Checklist
Choose a battery manufacturer who:
- Holds ISO 9001 + ISO 14001 + ISO 13485
- Has in-house cell aging test lab
- Provides custom BMS design
- Supports UN38.3 / IEC62133 certification
- Has long-term manufacturing consistency
12. Extended FAQ
Q1. What is the lifespan of a medical battery?
Typically 500–2000 cycles depending on chemistry.
Q2. Can consumer Li-ion cells be used for medical devices?
No. Medical-grade cells must be traceable, stable, and certified.
Q3. Which chemistry is safest?
LFP is the safest but not always suitable due to lower energy density.
Q4. Do medical batteries need UN38.3?
Yes, for all international transportation.
Q5. Do you support custom medical battery development?
Yes. A&S Power provides turnkey solutions from cell selection to certification.