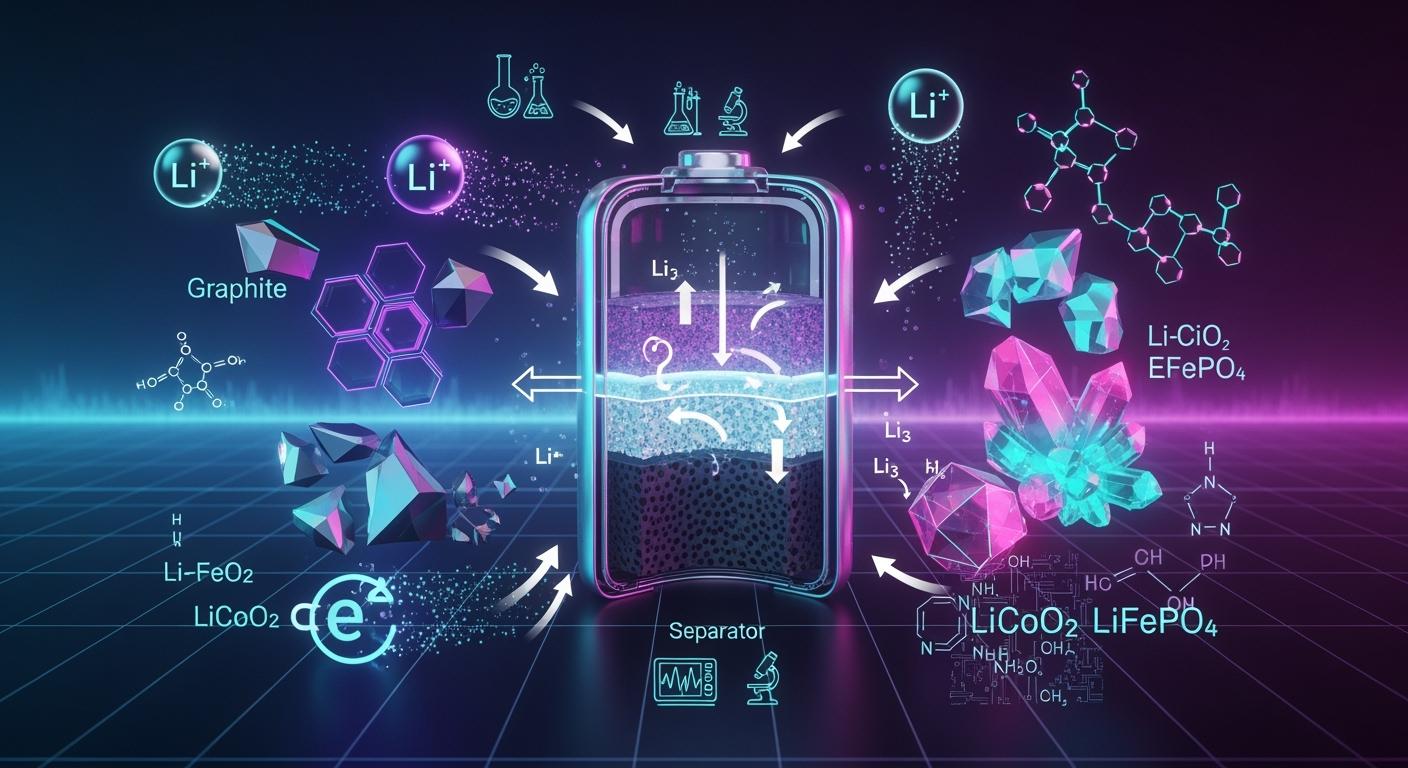
Materials and chemistry of lithium-ion batteries
You can find many important materials in a lithium-ion battery. These materials include lithium, cobalt, nickel, manganese, graphite, and a liquid or gel electrolyte. Each material plays a crucial role in helping the battery store and release energy safely. High-quality materials contribute to the longevity and performance of lithium-ion batteries. Additionally, power battery designs incorporate a flexible circuit board in power battery systems. This board is essential for controlling charging and ensuring the battery's safety. Scientists are actively researching new materials and recycling methods to enhance battery safety and minimize environmental impact.
Key Takeaways
- Lithium-ion batteries have important materials like lithium, cobalt, and graphite. These materials help the battery store and give out energy well.
- The battery has four main parts. These are the cathode, anode, separator, and electrolyte. Each part is important for storing energy and keeping the battery safe.
- Picking the right battery chemistry is very important for how well it works. Different things need different kinds of batteries.
- Recycling lithium-ion batteries helps the planet. It cuts down on waste and saves useful materials.
- Safety matters a lot. Always recycle batteries that are broken. Keep batteries away from heat so they do not catch fire.
Battery Structure and Function

Battery Components
Li-ion batteries have four main parts inside. Each part does something important. The cathode, anode, separator, and electrolyte all work together. They help the battery store and give out energy. The table below explains what each part does:
| Component | Role |
|---|---|
| Cathode (+) | A lithium compound that helps accept lithium ions when the battery gives out energy. |
| Anode (-) | Usually made of graphite, it lets go of lithium ions to the cathode during use. |
| Separator | Lets ions move but keeps the anode and cathode from touching each other. |
| Electrolyte | Moves lithium ions between the anode and cathode through the separator. |
Every part in li-ion batteries has a job. The cathode and anode are called electrodes. These electrodes hold and let go of lithium ions. The separator keeps the battery safe. It stops the electrodes from touching. The electrolyte lets ions move between the electrodes.
Rechargeable Chemistry
Li-ion batteries can be used many times. This is because of their special chemistry. When you charge the battery, lithium ions move to the anode. The anode keeps these ions safe. When you use the battery, the ions go back to the cathode. This movement is called intercalation. The electrodes do not change shape or break apart. This helps li-ion batteries last a long time.
Ion Movement in Lithium-Ion Batteries
Ion movement is very important in li-ion batteries. When you use the battery, lithium ions leave the anode. They travel through the electrolyte. The ions pass the separator and reach the cathode. Electrons move through a wire outside the battery. This gives you power. When you charge the battery, the ions go back to the anode. This cycle happens over and over. The electrodes must stay strong for the battery to work well. The design of li-ion batteries helps them last and stay safe.
Materials and Chemistries in Lithium-Ion Batteries

Common Materials in Li-Ion Batteries
Li-ion batteries have many important materials inside. Each material helps the battery work and stay safe. The most common ones are lithium, cobalt, nickel, manganese, graphite, titanium, and the electrolyte. Lithium is the main element. It moves between electrodes and stores energy. Cobalt and nickel help the battery hold more energy and last longer. Manganese makes the battery safer and lowers the cost. Graphite forms the anode and gives good cycle stability. Titanium sometimes replaces graphite in special batteries for better safety. The electrolyte lets lithium ions move between the anode and cathode.
You can check the table below to see the most used cathode and anode materials in commercial lithium-ion batteries. The table lists their types and main properties:
| Material | Type | Key Properties |
|---|---|---|
| Lithium Cobalt Oxide | Cathode | High energy density, good thermal stability |
| Graphite | Anode | High conductivity, good cycle stability |
| Lithium Manganese Oxide | Cathode | Lower cost, good thermal stability, safer |
| Lithium Iron Phosphate | Cathode | Long cycle life, thermal stability, lower energy density |
Each material gives the battery special strengths. Lithium cobalt oxide has high energy density. Lithium manganese oxide is safer and costs less. Lithium iron phosphate lasts a long time. Graphite helps the battery recharge many times.
Application-Specific Battery Chemistries
Different battery chemistries are used for different things. The chemistry changes based on what you need the battery to do. Electric cars need batteries with high energy density and long life. These batteries often use lithium nickel manganese cobalt oxide (NMC) or lithium iron phosphate. Phones and laptops use lithium cobalt oxide because it has high energy density. Power tools need batteries that give high power quickly. These tools use lithium manganese oxide or lithium titanate.
Battery chemistry affects how well the battery works in each situation. Some chemistries give more power. Others last longer or are safer. You pick the right chemistry for your battery to get the best results.
Tip: You can make batteries work better by choosing the right chemistry for your needs. Think about energy density, safety, and cycle life when you pick a battery.
Critical Minerals and Supply Issues
Li-ion batteries need important minerals like lithium, cobalt, and nickel. These minerals come from only a few places in the world. Sometimes, there are supply problems because mining and processing take time and cost money. Cobalt often comes from countries with limited resources and strict rules. Nickel and lithium also face supply challenges as demand grows.
Supply issues can change battery prices and how easy they are to get. Recycling old batteries helps get these minerals back and reduces waste. Scientists study new chemistries to use less cobalt and nickel. This research helps make batteries safer, cheaper, and better for the environment.
You should think about sustainability when using lithium-ion batteries. Recycling and new chemistry solutions help protect resources and keep battery technology moving forward.
Advantages and Disadvantages of Li-Ion Batteries
Performance and Energy Density
People pick li-ion batteries because they work well. These batteries hold a lot of energy in a small space. This makes them good for phones, laptops, and electric cars. The table below shows how long they last and their voltage:
| Battery Type | Cycle Life (cycles) | Nominal Voltage (V) |
|---|---|---|
| Lithium-ion (Li-ion) | 500 | 3.7 |
Li-ion batteries last longer and have higher voltage than older batteries. You get more power and your devices run longer before charging. The high voltage helps run strong electronics. If you need good energy storage, li-ion batteries give you great performance and long life.
Safety and Environmental Impact
Li-ion batteries work well, but safety is important. These batteries can catch fire if they get damaged or too hot. Sometimes, batteries swell or start fires when recycled or thrown away. The table below lists some safety problems:
| Safety Risk | Description |
|---|---|
| Fire hazards during recycling | Li-ion batteries can get crushed in recycling bins and may cause fires. |
| Fires during transport or disposal | Throwing them away wrong can start fires at landfills or during transport. |
| Swollen batteries as fire hazards | Swelling means damage and can lead to fires; handle with care. |
Never put a damaged or swollen li-ion battery in the trash. Use special places for recycling. These batteries use minerals like lithium, cobalt, and nickel. Getting these minerals can hurt nature. Recycling helps save resources and cuts down on waste. You help the earth when you recycle batteries and pick safer products.
Note: Keep your batteries in a cool, dry spot. Do not use damaged batteries to lower fire risk.
Cost and Scalability
Li-ion batteries cost more than old battery types. But they last longer and work better. Their price depends on materials like lithium and cobalt. Sometimes, prices go up if there are supply problems. More people want electric cars and clean energy, so demand is rising. Companies try to make batteries cheaper and easier to build.
You can find li-ion batteries in many sizes. Factories make tiny ones for earbuds and big ones for cars and solar power. This makes it easy to get the right battery for your device. As recycling gets better and new materials are found, prices may drop and batteries will improve.
Tip: To save money and help the planet, buy products with recycled materials or advanced battery chemistries.
Li-ion batteries give you strong power, lots of energy, and many choices. Handle them safely and recycle them to enjoy their benefits and protect nature.
Innovations and Battery Recycling
Research in Battery Materials
Scientists work on new battery ideas every year. They want li-ion batteries to be safer and last longer. They also want batteries to store more energy. Some teams use less cobalt in cathode materials. Others test silicon or lithium metal for the anode. These changes help batteries charge faster. They also help batteries work in hot or cold weather. Solid-state batteries use a solid electrolyte. This can lower fire risks and make batteries last longer.
Advanced Diagnostic Tools
Modern tools help us learn about li-ion batteries. Engineers use sensors and computer models to watch batteries. These tools find problems early, like overheating. They also spot when batteries lose power. Some systems use artificial intelligence to predict battery failure. This helps keep devices safe and working longer. Good diagnostics tell you when to recycle batteries.
Battery Recycling and Resource Conservation
Recycling li-ion batteries helps the planet. It keeps toxic materials out of landfills. It also saves important resources. The table below shows how recycling helps the environment and saves resources:
| Category | Evidence |
|---|---|
| Environmental Protection | Stops soil and water from getting toxic lead and sulfuric acid. |
| Cuts down on dangerous waste in landfills. | |
| Resource Conservation | Gets back lead, plastic, and acid for new batteries. |
| Lowers the need for new lead mining and saves nature. |
Recycling reduces pollution and helps recover metals. These metals include lithium, cobalt, and nickel. This means less mining and less damage to nature. Recycling old batteries helps make new ones. Safe disposal keeps your community clean and healthy.
Tip: Always take used li-ion batteries to a recycling center. Never put them in the trash.
You now know that every battery has special materials inside. These materials help the battery store and give out energy. The table below shows how each material helps the battery work well:
| Material Type | Role in Battery | Advantages |
|---|---|---|
| Lithium Cobalt Oxide | Cathode | Holds lots of energy, used in electronics |
| Graphite | Anode | Holds charge, can be used many times |
| Lithium Manganese Oxide | Cathode | Used in cars, safer to use |
| Lithium Iron Phosphate | Cathode | Very stable, safe, used in many things |
Scientists keep working on new battery ideas. Some new batteries use silicon-based anodes for better results. You can help the earth by recycling batteries and picking safe products. If you want to know more about battery safety, check the Huawei and ITU white paper. If you have questions, you can email us or use our contact form.
-

 May.2026.02.27Lithium-Ion Batteries: The Six Constraints Blocking the Path to PerfectionLearn More
May.2026.02.27Lithium-Ion Batteries: The Six Constraints Blocking the Path to PerfectionLearn More -

 May.2026.02.25Li-Polymer Battery 5000mAh: Complete Technical & OEM GuideLearn More
May.2026.02.25Li-Polymer Battery 5000mAh: Complete Technical & OEM GuideLearn More -

 May.2026.02.24The Unparalleled Advantages of Lithium-Ion Batteries Over Traditional BatteriesLearn More
May.2026.02.24The Unparalleled Advantages of Lithium-Ion Batteries Over Traditional BatteriesLearn More -

 May.2026.02.243.6 Volt Battery: Complete Technical Guide for Engineers & BuyersLearn More
May.2026.02.243.6 Volt Battery: Complete Technical Guide for Engineers & BuyersLearn More -

 May.2026.02.24What Is a 3.8V LiPo Battery? A Complete Engineering & OEM GuideLearn More
May.2026.02.24What Is a 3.8V LiPo Battery? A Complete Engineering & OEM GuideLearn More